What Type of Study Design Is a Systematic Review
- Review
- Open Access
- Published:
A pace past step guide for conducting a systematic review and meta-analysis with simulation information
Tropical Medicine and Health book 47, Article number:46 (2019) Cite this article
Abstract
Background
The massive abundance of studies relating to tropical medicine and health has increased strikingly over the last few decades. In the field of tropical medicine and health, a well-conducted systematic review and meta-analysis (SR/MA) is considered a feasible solution for keeping clinicians beside of current evidence-based medicine. Understanding of SR/MA steps is of paramount importance for its conduction. It is not easy to be washed as there are obstacles that could face the researcher. To solve those hindrances, this methodology written report aimed to provide a pace-past-step approach mainly for beginners and junior researchers, in the field of tropical medicine and other wellness care fields, on how to properly deport a SR/MA, in which all the steps here depicts our feel and expertise combined with the already well-known and accustomed international guidance.
We suggest that all steps of SR/MA should exist done independently past 2–three reviewers' discussion, to ensure data quality and accuracy.
Conclusion
SR/MA steps include the development of research question, forming criteria, search strategy, searching databases, protocol registration, title, abstract, total-text screening, manual searching, extracting data, quality assessment, data checking, statistical analysis, double data checking, and manuscript writing.
Introduction
The amount of studies published in the biomedical literature, especially tropical medicine and health, has increased strikingly over the last few decades. This massive abundance of literature makes clinical medicine increasingly circuitous, and cognition from various researches is often needed to inform a particular clinical determination. Even so, bachelor studies are frequently heterogeneous with regard to their blueprint, operational quality, and subjects under study and may handle the research question in a dissimilar mode, which adds to the complexity of bear witness and determination synthesis [i].
Systematic review and meta-analyses (SR/MAs) have a loftier level of evidence equally represented by the testify-based pyramid. Therefore, a well-conducted SR/MA is considered a viable solution in keeping health clinicians ahead regarding contemporary evidence-based medicine.
Differing from a systematic review, unsystematic narrative review tends to be descriptive, in which the authors select often articles based on their point of view which leads to its poor quality. A systematic review, on the other hand, is divers as a review using a systematic method to summarize bear witness on questions with a detailed and comprehensive plan of study. Furthermore, despite the increasing guidelines for effectively conducting a systematic review, we institute that bones steps ofttimes start from framing question, then identifying relevant piece of work which consists of criteria evolution and search for articles, appraise the quality of included studies, summarize the prove, and translate the results [ii, 3]. However, those unproblematic steps are not easy to exist reached in reality. There are many troubles that a researcher could be struggled with which has no detailed indication.
Conducting a SR/MA in tropical medicine and health may exist difficult especially for young researchers; therefore, agreement of its essential steps is crucial. It is not easy to be washed as there are obstacles that could face the researcher. To solve those hindrances, we recommend a flow diagram (Fig. 1) which illustrates a detailed and footstep-past-step the stages for SR/MA studies. This methodology study aimed to provide a stride-by-footstep arroyo mainly for beginners and inferior researchers, in the field of tropical medicine and other health care fields, on how to properly and succinctly conduct a SR/MA; all the steps here depicts our experience and expertise combined with the already well known and accustomed international guidance.
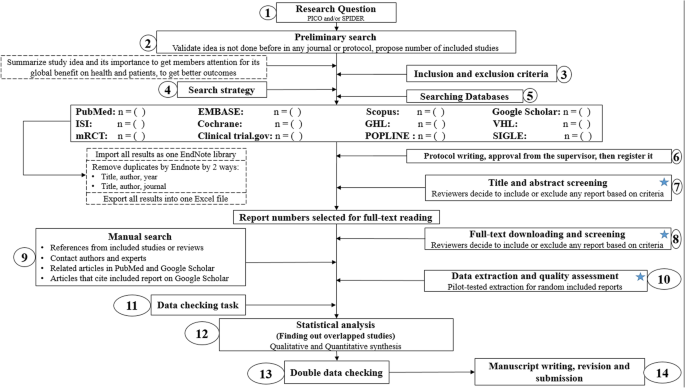
Detailed menstruation diagram guideline for systematic review and meta-analysis steps. Notation: Star icon refers to "two–3 reviewers screen independently"
Methods and results
Detailed steps for conducting whatsoever systematic review and meta-assay
We searched the methods reported in published SR/MA in tropical medicine and other healthcare fields besides the published guidelines like Cochrane guidelines {Higgins, 2011 #vii} [4] to collect the best low-bias method for each step of SR/MA conduction steps. Furthermore, we used guidelines that nosotros use in studies for all SR/MA steps. We combined these methods in society to conclude and conduct a detailed flow diagram that shows the SR/MA steps how beingness conducted.
Any SR/MA must follow the widely accepted Preferred Reporting Items for Systematic Review and Meta-analysis statement (PRISMA checklist 2009) (Additional file 5: Tabular array S1) [5].
We proposed our methods according to a valid explanatory simulation example choosing the topic of "evaluating safety of Ebola vaccine," equally it is known that Ebola is a very rare tropical disease but fatal. All the explained methods feature the standards followed internationally, with our compiled experience in the conduct of SR beside it, which we recollect proved some validity. This is a SR under conduct past a couple of researchers teaming in a research group, moreover, as the outbreak of Ebola which took place (2013–2016) in Africa resulted in a significant mortality and morbidity. Furthermore, since in that location are many published and ongoing trials assessing the safety of Ebola vaccines, nosotros idea this would provide a great opportunity to tackle this hotly debated issue. Moreover, Ebola started to fire again and new fatal outbreak appeared in the Democratic Republic of Congo since August 2018, which caused infection to more than 1000 people according to the Earth Health System, and 629 people have been killed till now. Hence, information technology is considered the 2d worst Ebola outbreak, subsequently the first one in Due west Africa in 2014, which infected more than than 26,000 and killed about 11,300 people forth outbreak course.
Research question and objectives
Like other study designs, the inquiry question of SR/MA should be feasible, interesting, novel, ethical, and relevant. Therefore, a articulate, logical, and well-defined research question should be formulated. Usually, two mutual tools are used: PICO or SPIDER. PICO (Population, Intervention, Comparing, Effect) is used mostly in quantitative show synthesis. Authors demonstrated that PICO holds more sensitivity than the more specific SPIDER arroyo [half-dozen]. SPIDER (Sample, Phenomenon of Interest, Pattern, Evaluation, Research blazon) was proposed equally a method for qualitative and mixed methods search.
Nosotros hither recommend a combined approach of using either one or both the SPIDER and PICO tools to recollect a comprehensive search depending on time and resource limitations. When nosotros apply this to our assumed research topic, being of qualitative nature, the employ of SPIDER approach is more valid.
PICO is usually used for systematic review and meta-analysis of clinical trial report. For the observational study (without intervention or comparator), in many tropical and epidemiological questions, it is commonly enough to use P (Patient) and O (issue) simply to formulate a inquiry question. We must bespeak conspicuously the population (P), and then intervention (I) or exposure. Next, it is necessary to compare (C) the indicated intervention with other interventions, i.e., placebo. Finally, nosotros need to clarify which are our relevant outcomes.
To facilitate comprehension, we cull the Ebola virus disease (EVD) as an example. Currently, the vaccine for EVD is being adult and under phase I, II, and III clinical trials; nosotros want to know whether this vaccine is prophylactic and can induce sufficient immunogenicity to the subjects.
An example of a research question for SR/MA based on PICO for this event is as follows: How is the safety and immunogenicity of Ebola vaccine in homo? (P: healthy subjects (man), I: vaccination, C: placebo, O: safety or agin effects)
Preliminary research and thought validation
We recommend a preliminary search to identify relevant manufactures, ensure the validity of the proposed idea, avert duplication of previously addressed questions, and clinch that we have plenty articles for conducting its assay. Moreover, themes should focus on relevant and important health-care problems, consider global needs and values, reflect the electric current science, and be consistent with the adopted review methods. Gaining familiarity with a deep understanding of the written report field through relevant videos and discussions is of paramount importance for ameliorate retrieval of results. If we ignore this footstep, our study could exist canceled whenever we find out a similar study published earlier. This means nosotros are wasting our time to deal with a problem that has been tackled for a long time.
To exercise this, we can starting time past doing a elementary search in PubMed or Google Scholar with search terms Ebola AND vaccine. While doing this step, we place a systematic review and meta-analysis of determinant factors influencing antibiotic response from vaccination of Ebola vaccine in non-human primate and human [seven], which is a relevant paper to read to become a deeper insight and identify gaps for improve formulation of our inquiry question or purpose. We can notwithstanding carry systematic review and meta-analysis of Ebola vaccine because we evaluate safety equally a dissimilar outcome and different population (only homo).
Inclusion and exclusion criteria
Eligibility criteria are based on the PICO approach, study design, and date. Exclusion criteria more often than not are unrelated, duplicated, unavailable full texts, or abstract-merely papers. These exclusions should be stated in accelerate to refrain the researcher from bias. The inclusion criteria would be articles with the target patients, investigated interventions, or the comparing between two studied interventions. Briefly, information technology would be manufactures which contain data answering our research question. But the well-nigh important is that it should be clear and sufficient information, including positive or negative, to answer the question.
For the topic we have chosen, nosotros can make inclusion criteria: (i) any clinical trial evaluating the safety of Ebola vaccine and (two) no restriction regarding country, patient historic period, race, gender, publication language, and date. Exclusion criteria are equally follows: (1) written report of Ebola vaccine in non-human subjects or in vitro studies; (ii) study with data non reliably extracted, duplicate, or overlapping data; (3) abstract-only papers as preceding papers, conference, editorial, and writer response theses and books; (4) articles without available full text available; and (v) example reports, instance series, and systematic review studies. The PRISMA period diagram template that is used in SR/MA studies can be establish in Fig. 2.
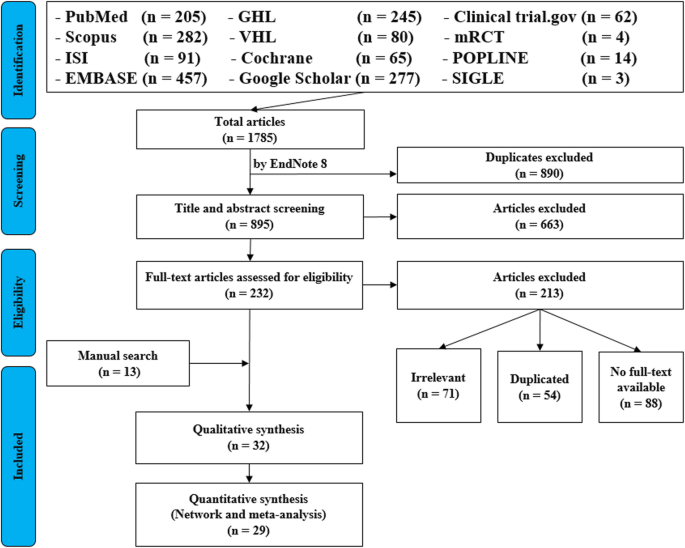
PRISMA menstruum diagram of studies' screening and selection
Search strategy
A standard search strategy is used in PubMed, then later it is modified according to each specific database to get the best relevant results. The basic search strategy is built based on the research question formulation (i.e., PICO or PICOS). Search strategies are synthetic to include free-text terms (e.chiliad., in the title and abstract) and any advisable subject indexing (eastward.g., MeSH) expected to retrieve eligible studies, with the help of an expert in the review topic field or an data specialist. Additionally, we advise non to apply terms for the Outcomes every bit their inclusion might hinder the database being searched to recall eligible studies because the used outcome is non mentioned obviously in the articles.
The improvement of the search term is made while doing a trial search and looking for some other relevant term within each concept from retrieved papers. To search for a clinical trial, we can utilize these descriptors in PubMed: "clinical trial"[Publication Type] OR "clinical trials as topic"[MeSH terms] OR "clinical trial"[All Fields]. Afterwards some rounds of trial and refinement of search term, we codify the final search term for PubMed equally follows: (ebola OR ebola virus OR ebola virus affliction OR EVD) AND (vaccine OR vaccination OR vaccinated OR immunization) AND ("clinical trial"[Publication Type] OR "clinical trials every bit topic"[MeSH Terms] OR "clinical trial"[All Fields]). Because the study for this topic is express, we do not include outcome term (safety and immunogenicity) in the search term to capture more than studies.
Search databases, import all results to a library, and exporting to an excel sheet
According to the AMSTAR guidelines, at least two databases have to exist searched in the SR/MA [8], but as y'all increase the number of searched databases, yous get much yield and more accurate and comprehensive results. The ordering of the databases depends mostly on the review questions; being in a study of clinical trials, you will rely mostly on Cochrane, mRCTs, or International Clinical Trials Registry Platform (ICTRP). Hither, we propose 12 databases (PubMed, Scopus, Web of Science, EMBASE, GHL, VHL, Cochrane, Google Scholar, Clinical trials.gov, mRCTs, POPLINE, and SIGLE), which help to cover nearly all published articles in tropical medicine and other health-related fields. Amidst those databases, POPLINE focuses on reproductive wellness. Researchers should consider to choose relevant database co-ordinate to the research topic. Some databases practice not support the use of Boolean or quotation; otherwise, there are some databases that have special searching way. Therefore, we need to modify the initial search terms for each database to become appreciated results; therefore, manipulation guides for each online database searches are presented in Additional file 5: Table S2. The detailed search strategy for each database is plant in Additional file 5: Table S3. The search term that we created in PubMed needs customization based on a specific feature of the database. An case for Google Scholar advanced search for our topic is as follows:
- one.
With all of the words: ebola virus
With at least one of the words: vaccine vaccination vaccinated immunization
Where my words occur: in the title of the article
- 2.
With all of the words: EVD
With at to the lowest degree one of the words: vaccine vaccination vaccinated immunization
Where my words occur: in the title of the commodity
Finally, all records are collected into one Endnote library in order to delete duplicates and then to it export into an excel sheet. Using remove duplicating function with two options is mandatory. All references which have (one) the same championship and author, and published in the aforementioned year, and (2) the same title and writer, and published in the same journal, would be deleted. References remaining after this stride should be exported to an excel file with essential data for screening. These could be the authors' names, publication year, journal, DOI, URL link, and abstruse.
Protocol writing and registration
Protocol registration at an early on stage guarantees transparency in the research process and protects from duplication problems. Besides, it is considered a documented proof of team programme of action, enquiry question, eligibility criteria, intervention/exposure, quality assessment, and pre-analysis program. It is recommended that researchers send it to the main investigator (PI) to revise it, then upload it to registry sites. There are many registry sites available for SR/MA similar those proposed by Cochrane and Campbell collaborations; however, we recommend registering the protocol into PROSPERO every bit information technology is easier. The layout of a protocol template, co-ordinate to PROSPERO, tin can be found in Additional file v: File S1.
Title and abstract screening
Decisions to select retrieved articles for further assessment are based on eligibility criteria, to minimize the chance of including non-relevant articles. According to the Cochrane guidance, two reviewers are a must to exercise this step, just as for beginners and junior researchers, this might be deadening; thus, we propose based on our feel that at to the lowest degree 3 reviewers should work independently to reduce the chance of error, particularly in teams with a big number of authors to add more scrutiny and ensure proper carry. Mostly, the quality with 3 reviewers would be amend than ii, as two only would have unlike opinions from each other, then they cannot make up one's mind, while the tertiary opinion is crucial. And here are some examples of systematic reviews which we conducted following the same strategy (by a different group of researchers in our research group) and published successfully, and they characteristic relevant ideas to tropical medicine and affliction [9,10,xi].
In this step, duplications will be removed manually whenever the reviewers find them out. When there is a doubt about an article decision, the squad should be inclusive rather than exclusive, until the main leader or PI makes a decision afterward give-and-take and consensus. All excluded records should be given exclusion reasons.
Full text downloading and screening
Many search engines provide links for gratis to access full-text articles. In case non found, we can search in some enquiry websites equally ResearchGate, which offering an choice of straight full-text request from authors. Additionally, exploring athenaeum of wanted journals, or contacting PI to purchase it if available. Similarly, ii–3 reviewers work independently to make up one's mind about included total texts co-ordinate to eligibility criteria, with reporting exclusion reasons of articles. In case any disagreement has occurred, the terminal conclusion has to be made by word.
Manual search
I has to exhaust all possibilities to reduce bias past performing an explicit hand-searching for retrieval of reports that may accept been dropped from outset search [12]. We apply 5 methods to make transmission searching: searching references from included studies/reviews, contacting authors and experts, and looking at related articles/cited manufactures in PubMed and Google Scholar.
Nosotros draw here three consecutive methods to increase and refine the yield of manual searching: firstly, searching reference lists of included articles; secondly, performing what is known equally citation tracking in which the reviewers rail all the articles that cite each one of the included articles, and this might involve electronic searching of databases; and thirdly, similar to the citation tracking, nosotros follow all "related to" or "similar" articles. Each of the abovementioned methods can be performed by 2–3 independent reviewers, and all the possible relevant article must undergo further scrutiny against the inclusion criteria, after following the same records yielded from electronic databases, i.e., title/abstract and full-text screening.
We advise an contained reviewing past assigning each fellow member of the teams a "tag" and a distinct method, to compile all the results at the end for comparison of differences and word and to maximize the retrieval and minimize the bias. Similarly, the number of included articles has to be stated earlier add-on to the overall included records.
Data extraction and quality assessment
This step entitles data collection from included full-texts in a structured extraction excel sheet, which is previously airplane pilot-tested for extraction using some random studies. We recommend extracting both adjusted and not-adjusted data because it gives the almost allowed confounding factor to exist used in the analysis by pooling them later on [13]. The procedure of extraction should be executed past 2–iii independent reviewers. More often than not, the sheet is classified into the written report and patient characteristics, outcomes, and quality cess (QA) tool.
Information presented in graphs should be extracted by software tools such every bit Web plot digitizer [14]. Nigh of the equations that can be used in extraction prior to assay and estimation of standard deviation (SD) from other variables is found within Additional file 5: File S2 with their references equally Hozo et al. [15], Xiang et al. [16], and Rijkom et al. [17]. A variety of tools are available for the QA, depending on the design: ROB-2 Cochrane tool for randomized controlled trials [eighteen] which is presented equally Boosted file 1: Figure S1 and Additional file 2: Figure S2—from a previous published article data—[xix], NIH tool for observational and cross-sectional studies [20], ROBINS-I tool for non-randomize trials [21], QUADAS-2 tool for diagnostic studies, QUIPS tool for prognostic studies, CARE tool for instance reports, and ToxRtool for in vivo and in vitro studies. Nosotros recommend that 2–iii reviewers independently assess the quality of the studies and add to the information extraction form earlier the inclusion into the analysis to reduce the gamble of bias. In the NIH tool for observational studies—cohort and cantankerous-sectional—as in this EBOLA case, to evaluate the risk of bias, reviewers should rate each of the xiv items into dichotomous variables: yeah, no, or not applicative. An overall score is calculated by calculation all the items scores as aye equals ane, while no and NA equals naught. A score volition be given for every newspaper to classify them as poor, off-white, or adept conducted studies, where a score from 0–5 was considered poor, six–nine as fair, and 10–xiv every bit adept.
In the EBOLA case example above, authors tin extract the post-obit information: name of authors, state of patients, year of publication, study design (instance study, cohort study, or clinical trial or RCT), sample size, the infected indicate of time after EBOLA infection, follow-upwards interval after vaccination time, efficacy, condom, adverse effects later on vaccinations, and QA sheet (Additional file half dozen: Data S1).
Data checking
Due to the expected human mistake and bias, we recommend a information checking pace, in which every included commodity is compared with its counterpart in an extraction canvas past evidence photos, to detect mistakes in information. We suggest assigning articles to two–3 independent reviewers, ideally not the ones who performed the extraction of those articles. When resource are limited, each reviewer is assigned a unlike article than the one he extracted in the previous phase.
Statistical analysis
Investigators use different methods for combining and summarizing findings of included studies. Before analysis, there is an important step called cleaning of information in the extraction canvass, where the analyst organizes extraction sail data in a form that can be read by analytical software. The assay consists of 2 types namely qualitative and quantitative analysis. Qualitative analysis mostly describes information in SR studies, while quantitative analysis consists of two main types: MA and network meta-analysis (NMA). Subgroup, sensitivity, cumulative analyses, and meta-regression are appropriate for testing whether the results are consistent or not and investigating the outcome of certain confounders on the outcome and finding the all-time predictors. Publication bias should be assessed to investigate the presence of missing studies which tin can affect the summary.
To illustrate bones meta-analysis, we provide an imaginary data for the inquiry question well-nigh Ebola vaccine safety (in terms of agin events, 14 days after injection) and immunogenicity (Ebola virus antibodies rise in geometric mean titer, six months later on injection). Assuming that from searching and information extraction, we decided to practice an analysis to evaluate Ebola vaccine "A" safety and immunogenicity. Other Ebola vaccines were not meta-analyzed because of the limited number of studies (instead, it will be included for narrative review). The imaginary data for vaccine condom meta-analysis can be accessed in Boosted file vii: Data S2. To do the meta-analysis, we can apply costless software, such every bit RevMan [22] or R bundle meta [23]. In this example, nosotros volition utilize the R parcel meta. The tutorial of meta packet tin can be accessed through "Full general Packet for Meta-Analysis" tutorial pdf [23]. The R codes and its guidance for meta-analysis washed tin be institute in Boosted file v: File S3.
For the analysis, we assume that the written report is heterogenous in nature; therefore, we choose a random effect model. We did an analysis on the condom of Ebola vaccine A. From the data table, we can run across some adverse events occurring after intramuscular injection of vaccine A to the field of study of the study. Suppose that we include six studies that fulfill our inclusion criteria. We can practise a meta-analysis for each of the adverse events extracted from the studies, for example, arthralgia, from the results of random issue meta-analysis using the R meta package.
From the results shown in Additional file iii: Effigy S3, nosotros tin can come across that the odds ratio (OR) of arthralgia is 1.06 (0.79; i.42), p value = 0.71, which ways that there is no association between the intramuscular injection of Ebola vaccine A and arthralgia, as the OR is almost one, and besides, the P value is insignificant every bit it is > 0.05.
In the meta-assay, we can as well visualize the results in a forest plot. Information technology is shown in Fig. 3 an instance of a forest plot from the simulated analysis.
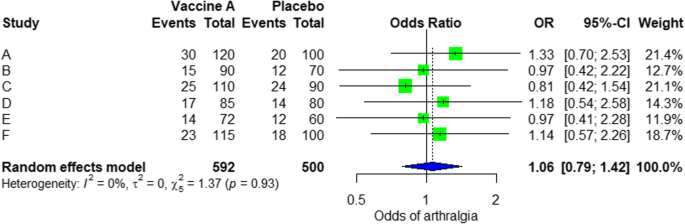
Random event model forest plot for comparing of vaccine A versus placebo
From the woods plot, we tin can see six studies (A to F) and their respective OR (95% CI). The green box represents the consequence size (in this case, OR) of each study. The bigger the box means the study weighted more (i.due east., bigger sample size). The bluish diamond shape represents the pooled OR of the six studies. We can see the bluish diamond cross the vertical line OR = ane, which indicates no significance for the association as the diamond well-nigh equalized in both sides. Nosotros can confirm this also from the 95% confidence interval that includes one and the p value > 0.05.
For heterogeneity, we run into that I 2 = 0%, which means no heterogeneity is detected; the study is relatively homogenous (information technology is rare in the real study). To evaluate publication bias related to the meta-analysis of adverse events of arthralgia, we can apply the metabias function from the R meta packet (Additional file 4: Figure S4) and visualization using a funnel plot. The results of publication bias are demonstrated in Fig. four. Nosotros run into that the p value associated with this test is 0.74, indicating symmetry of the funnel plot. We tin can confirm it by looking at the funnel plot.
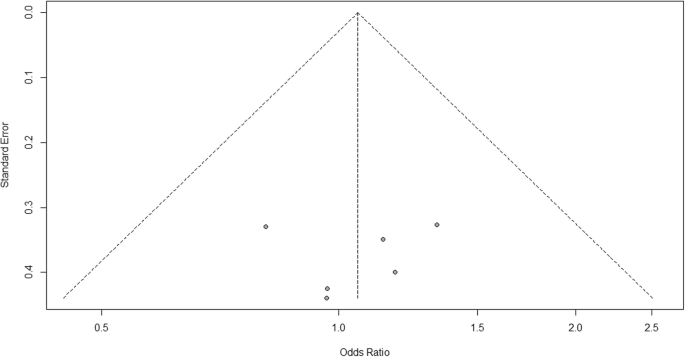
Publication bias funnel plot for comparison of vaccine A versus placebo
Looking at the funnel plot, the number of studies at the left and right side of the funnel plot is the same; therefore, the plot is symmetry, indicating no publication bias detected.
Sensitivity analysis is a procedure used to discover how different values of an independent variable will influence the significance of a particular dependent variable by removing ane study from MA. If all included study p values are < 0.05, hence, removing whatsoever written report will not change the significant clan. It is only performed when at that place is a significant association, then if the p value of MA done is 0.7—more than i—the sensitivity analysis is not needed for this case report example. If there are 2 studies with p value > 0.05, removing any of the two studies will result in a loss of the significance.
Double data checking
For more assurance on the quality of results, the analyzed information should be rechecked from full-text data by prove photos, to allow an obvious check for the PI of the study.
Manuscript writing, revision, and submission to a journal
Writing based on four scientific sections: introduction, methods, results, and discussion, generally with a conclusion. Performing a characteristic tabular array for report and patient characteristics is a mandatory step which can exist found as a template in Additional file 5: Table S3.
After finishing the manuscript writing, characteristics table, and PRISMA menstruum diagram, the team should send it to the PI to revise it well and answer to his comments and, finally, choose a suitable journal for the manuscript which fits with considerable impact gene and plumbing equipment field. We need to pay attention by reading the author guidelines of journals before submitting the manuscript.
Give-and-take
The role of evidence-based medicine in biomedical enquiry is rapidly growing. SR/MAs are likewise increasing in the medical literature. This newspaper has sought to provide a comprehensive arroyo to enable reviewers to produce high-quality SR/MAs. Nosotros hope that readers could gain general knowledge about how to behave a SR/MA and take the conviction to perform one, although this kind of study requires complex steps compared to narrative reviews.
Having the bones steps for conduction of MA, there are many avant-garde steps that are applied for certain specific purposes. 1 of these steps is meta-regression which is performed to investigate the clan of any confounder and the results of the MA. Furthermore, there are other types rather than the standard MA like NMA and MA. In NMA, we investigate the difference between several comparisons when there were non enough information to enable standard meta-analysis. It uses both direct and indirect comparisons to conclude what is the best between the competitors. On the other mitt, mega MA or MA of patients tend to summarize the results of independent studies past using its individual discipline information. As a more detailed analysis can be done, information technology is useful in conducting repeated measure out analysis and fourth dimension-to-event analysis. Moreover, it can perform analysis of variance and multiple regression assay; all the same, it requires homogenous dataset and it is time-consuming in acquit [24].
Conclusions
Systematic review/meta-analysis steps include development of inquiry question and its validation, forming criteria, search strategy, searching databases, importing all results to a library and exporting to an excel sheet, protocol writing and registration, title and abstract screening, total-text screening, manual searching, extracting data and assessing its quality, data checking, conducting statistical analysis, double data checking, manuscript writing, revising, and submitting to a journal.
Availability of data and materials
Not applicable.
Abbreviations
- NMA:
-
Network meta-analysis
- PI:
-
Primary investigator
- PICO:
-
Population, Intervention, Comparison, Effect
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-assay statement
- QA:
-
Quality assessment
- SPIDER:
-
Sample, Phenomenon of Interest, Design, Evaluation, Research type
- SR/MAs:
-
Systematic review and meta-analyses
References
-
Bello A, Wiebe N, Garg A, Tonelli M. Bear witness-based decision-making 2: systematic reviews and meta-analysis. Methods Mol Biol (Clifton, NJ). 2015;1281:397–416.
-
Khan KS, Kunz R, Kleijnen J, Antes Chiliad. Five steps to conducting a systematic review. J R Soc Med. 2003;96(3):118–21.
-
Rys P, Wladysiuk M, Skrzekowska-Baran I, Malecki MT. Review articles, systematic reviews and meta-analyses: which can be trusted? Polskie Archiwum Medycyny Wewnetrznej. 2009;119(3):148–56.
-
Higgins JPT, Green South. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. 2011.
-
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
-
Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;fourteen:579.
-
Gross Fifty, Lhomme E, Pasin C, Richert L, Thiebaut R. Ebola vaccine development: systematic review of pre-clinical and clinical studies, and meta-assay of determinants of antibody response variability after vaccination. Int J Infect Dis. 2018;74:83–96.
-
Shea BJ, Reeves BC, Wells G, Thuku 1000, Hamel C, Moran J, ... Henry DA. AMSTAR two: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
-
Giang HTN, Banno K, Minh LHN, Trinh LT, Loc LT, Eltobgy A, et al. Dengue hemophagocytic syndrome: a systematic review and meta-assay on epidemiology, clinical signs, outcomes, and risk factors. Rev Med Virol. 2018;28(6):e2005.
-
Morra ME, Altibi AMA, Iqtadar S, Minh LHN, Elawady SS, Hallab A, et al. Definitions for warning signs and signs of severe dengue according to the WHO 2009 classification: systematic review of literature. Rev Med Virol. 2018;28(four):e1979.
-
Morra ME, Van Thanh L, Kamel MG, Ghazy AA, Altibi AMA, Dat LM, et al. Clinical outcomes of current medical approaches for Middle E respiratory syndrome: a systematic review and meta-analysis. Rev Med Virol. 2018;28(3):e1977.
-
Vassar Thousand, Atakpo P, Kash MJ. Manual search approaches used by systematic reviewers in dermatology. Periodical of the Medical Library Association: JMLA. 2016;104(4):302.
-
Naunheim MR, Remenschneider AK, Scangas GA, Bunting GW, Deschler DG. The event of initial tracheoesophageal voice prosthesis size on postoperative complications and voice outcomes. Ann Otol Rhinol Laryngol. 2016;125(6):478–84.
-
Rohatgi AJaiWa. Web Plot Digitizer. ht tp. 2014;two.
-
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13.
-
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard divergence from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135.
-
Van Rijkom HM, Truin GJ, Van't Hof MA. A meta-analysis of clinical studies on the caries-inhibiting effect of fluoride gel treatment. Carries Res. 1998;32(2):83–92.
-
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman Advertisement, et al. The Cochrane Collaboration's tool for assessing take chances of bias in randomised trials. BMJ. 2011;343:d5928.
-
Tawfik GM, Tieu TM, Ghozy S, Makram OM, Samuel P, Abdelaal A, et al. Speech efficacy, safety and factors affecting lifetime of voice prostheses in patients with laryngeal cancer: a systematic review and network meta-analysis of randomized controlled trials. J Clin Oncol. 2018;36(15_suppl):e18031-eastward.
-
Wannemuehler TJ, Lobo BC, Johnson JD, Deig CR, Ting JY, Gregory RL. Vibratory stimulus reduces in vitro biofilm formation on tracheoesophageal voice prostheses. Laryngoscope. 2016;126(12):2752–7.
-
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355.
-
RevMan The Cochrane Collaboration %J Copenhagen TNCCTCC. Review Director (RevMan). v.0. 2008.
-
Schwarzer GJRn. meta: An R package for meta-assay. 2007;vii(three):40-45.
-
Simms LLH. Meta-assay versus mega-analysis: is at that place a difference? Oral budesonide for the maintenance of remission in Crohn'southward disease: Kinesthesia of Graduate Studies, Academy of Western Ontario; 1998.
Funding
This study was conducted (in part) at the Joint Usage/Inquiry Center on Tropical Illness, Institute of Tropical Medicine, Nagasaki University, Japan.
Writer data
Affiliations
Contributions
NTH and GMT were responsible for the thought and its design. The figure was done by GMT. All authors contributed to the manuscript writing and approval of the final version.
Corresponding author
Ethics declarations
Ethics blessing and consent to participate
Not applicative.
Consent for publication
Non applicative.
Competing interests
The authors declare that they take no competing interests.
Additional data
Publisher'southward Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Effigy S1. Gamble of bias assessment graph of included randomized controlled trials. (TIF xx kb)
Additional file 2:
Figure S2. Risk of bias assessment summary. (TIF 69 kb)
Additional file 3:
Effigy S3. Arthralgia results of random consequence meta-analysis using R meta package. (TIF twenty kb)
Additional file 4:
Figure S4. Arthralgia linear regression test of funnel plot asymmetry using R meta bundle. (TIF 13 kb)
Boosted file 5:
Table S1. PRISMA 2009 Checklist. Tabular array S2. Manipulation guides for online database searches. Table S3. Detailed search strategy for twelve database searches. Table S4. Baseline characteristics of the patients in the included studies. File S1. PROSPERO protocol template file. File S2. Extraction equations that can be used prior to assay to become missed variables. File S3. R codes and its guidance for meta-assay done for comparison between EBOLA vaccine A and placebo. (DOCX 49 kb)
Additional file 6:
Data S1. Extraction and quality cess data sheets for EBOLA case example. (XLSX 1368 kb)
Additional file 7:
Information S2. Imaginary data for EBOLA case example. (XLSX 10 kb)
Rights and permissions
Open up Access This article is distributed under the terms of the Artistic Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you lot give appropriate credit to the original author(s) and the source, provide a link to the Creative Eatables license, and indicate if changes were made. The Artistic Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/cipher/1.0/) applies to the information fabricated available in this commodity, unless otherwise stated.
Reprints and Permissions
About this article
Cite this commodity
Tawfik, Yard.K., Dila, 1000.A.Southward., Mohamed, K.Y.F. et al. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop Med Health 47, 46 (2019). https://doi.org/ten.1186/s41182-019-0165-vi
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1186/s41182-019-0165-6
Keywords
- Search
- Information
- Extraction
- Assay
- Study
- Results
davalosliturmlime.blogspot.com
Source: https://tropmedhealth.biomedcentral.com/articles/10.1186/s41182-019-0165-6
0 Response to "What Type of Study Design Is a Systematic Review"
Post a Comment